What are Endometrial Hyperplastic Processes?
Intrauterine pathology includes endometrial hyperplastic processes; submucous uterine fibroids; adenomyosis (internal endometriosis); septum in the uterus; synechia; foreign bodies. In the structure of gynecological diseases, intrauterine pathology occupies a significant place.
Endometrial hyperplastic processes (GGE) are possible at any age, but their frequency increases significantly by the period of perimenopause. The increase in the incidence of GGE is associated both with an increase in life expectancy and with an unfavorable environmental situation, an increase in the number of chronic somatic diseases and a decrease in immunity in women.
Pathogenesis during Endometrial Hyperplastic Processes
The endometrium is a target organ for sex hormones due to the presence of specific receptors in it. A balanced hormonal effect through cytoplasmic and nuclear receptors provides physiological cyclic transformations of the uterine mucosa. Violation of hormonal status can lead to a change in the growth and differentiation of cellular elements of the endometrium and lead to the development of hyperplastic processes.
The leading place in the pathogenesis of HPE is given to relative or absolute hyperestrogenism, the absence of antiestrogenic effects of progesterone, or insufficient effects. Causes of hyperestrogenia: anovulation due to persistence or atresia of the follicles; hyperplastic processes in the ovaries or hormone-producing ovarian tumors (stromal hyperplasia, tekomatosis, granulosa cell tumor, theca cell tumors, etc.); violation of the pituitary gonadotropic function; hyperplasia of the adrenal cortex; improper use of hormonal drugs (estrogens, antiestrogens).
However, GGE can also develop with undisturbed hormonal ratios. In the development of such pathological processes, the leading role is given to disorders of tissue reception. Infectious and inflammatory changes in the endometrium can lead to the development of HPE in 30% of patients.
In the pathogenesis of GGE, exchange-endocrine disorders also play a large role: changes in fat metabolism, metabolism of sex hormones in the pathology of the hepatobiliary system and the gastrointestinal tract, immunity, and thyroid function.
Bohman put forward the concept of two pathogenetic variants of GGE. The first option is characterized by the diversity and depth of hyperestrogenism in combination with impaired fat and carbohydrate metabolism and is manifested in anovulatory uterine bleeding, infertility, late onset of menopause, ovarian tissue hyperplasia, in combination with feminizing ovarian tumors and polycystic ovary syndrome. Often, uterine fibroids and diffuse endometrial hyperplasia occur, against which polyps, foci of atypical endometrial hyperplasia and cancer occur. Metabolic disorders lead to obesity, hyperlipidemia and diabetes. In the second pathogenetic variant, these endocrine-metabolic disorders are not clearly expressed or even absent; Ovarian stromal fibrosis is combined with the normal structure or atrophy of the endometrium, with the appearance of polyps, focal hyperplasia (including atypical) and endometrial cancer.
In recent years, a complex system of factors involved in cell regulation has been identified, and ideas about intercellular interaction and intracellular processes in hormone-dependent tissues have been expanded. Thus, it has been established that along with estrogens, a number of biologically active compounds (polypeptide growth factors, cytokines, arachidonic acid metabolites), as well as a system of cellular and humoral immunity, are involved in the regulation of the proliferative activity of endometrial cells. In the regulation of tissue homeostasis and the pathogenesis of proliferative diseases, an important role belongs not only to increased cell proliferation, but also to dysregulation of cell death (apoptosis). Endometrial cell resistance to programmed cell death (apoptosis) leads to the accumulation of altered and excessively proliferating cells, which is characteristic of neoplastic changes in the endometrium.
Thus, the pathological transformation of the endometrium is a complex biological process that affects all parts of the neurohumoral regulation of a woman’s body.
Hyperplastic changes, as a rule, undergo the functional layer of the endometrium, much less often – the basal.
According to the histological classification of WHO (1975), 3 main types of GGE are distinguished: endometrial polyps, endometrial hyperplasia, atypical endometrial hyperplasia.
In 1994, WHO adopted a new classification of GGE, based on the recommendations of leading gynecologists and pathomorphologists.
- 1.1. GPE – proliferation of endometrial glands without cytological atypia:
- 1.1.1. Simple GGE corresponds to the glandular-cystic hyperplasia, adopted earlier, with the excess growth of the predominantly epithelial component of the glands characteristic of this condition.
- 1.1.2. Complex or complex (adenomatosis) GGE corresponds to atypical GGE of the 1st degree, differs from simple GGE in structural reorganization of glands and proliferation of epithelial glands.
- 1.2. Atypical GGE – proliferation of endometrial glands with signs of cytological atypia:
- 1.2.1. Simple atypical GGE corresponds to atypical GGE II degree and is characterized by pronounced proliferation of glandular epithelium without signs of cellular and nuclear polymorphism.
- 1.2.2. A complex or complex atypical GGE is similar to an atypical GGE of the III degree and has signs of cellular and nuclear polymorphism along with disorganization of the epithelium of the endometrial glands.
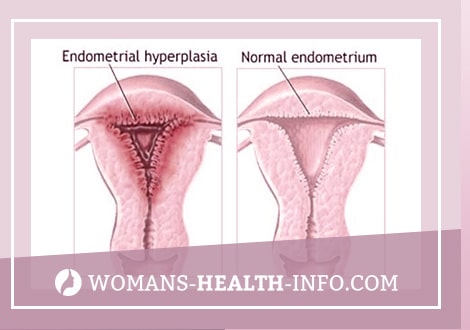
HPE occurs in approximately 5% of gynecological patients. This “histological” concept means increased growth, thickening of the uterine mucosa up to 15 mm or more, lack of separation into compact and spongy layers, violation of the correct distribution of glands in the stroma. Depending on the severity of proliferative processes, glandular HPE is divided into “active” and “resting”. The “active” form is characterized by a large number of mitoses in the epithelial cells of the glands and stroma, high alkaline phosphatase activity and the appearance of clusters of light cells in the glands. These signs indicate an intense estrogenic effect – absolute hyperestrogenism (follicle persistence). The “resting” form of glandular HPE occurs with prolonged exposure to the endometrium of low levels of estrogen hormones (follicular atresia). The endometrium is resting, nonfunctioning: the nuclei of the epithelium are intensely colored, mitoses are very rare or do not occur at all.
According to the state of the glands, glandular and glandular-cystic GGE are distinguished (B.I. Zheleznoye, 1981). In addition, distinguish between diffuse and focal, simple and polypoid GGE. However, most morphologists do not see a fundamental difference between them.
According to the classification of the International Association of Pathomorphologists (1985), GGE is divided into hyperplasia without cell atypia and hyperplasia with cell atypia, which is important for patient management tactics. Simple hyperplasia with minor structural disorders of the glands and complex (complex) with altered architectonics of the endometrium are distinguished.
An atypical GGE suggests a cytological atypia, namely the absence of polarity, an increase and stratification of nuclei, a change in their shape, an increase in the nuclear-plasma ratio, and irregular chromatin complexes.
By classification B.I. Zheleznova (1977), the terms “atypical” and “adenomatous” GGE are used as synonyms, but the first is used in relation to diffuse lesions of the endometrium, and the second – to local processes.
GGE is of interest mainly from the point of view of the development of endometrial cancer. Simple GGE without atypia goes into cancer in 1% of cases, polypoid without atypia – 3 times more often. Simple atypical GPE without treatment progresses to cancer in 8% of patients, and complex atypical GPE in 29% of patients.
From morphological positions, endometrial precancer includes hyperplasia with atypia (atypical HPE) and adenomatous polyps. However, the risk of malignancy of HPE depends not only on the morphological form, but also on the associated gynecological and extragenital pathology (polycystic ovary syndrome, feminizing ovarian tumors, uterine fibroids, obesity, diabetes mellitus or impaired glucose tolerance, hyperlipidemia, impaired hepatobiliary system function). G.M. Savelyev and V.N. Serov (1980) proposed a clinical-morphological classification according to which atypical HPE and adenomatous polyps in a woman of any age are referred to as endometrial precancer; recurrent glandular HPE in combination with hypothalamic and neuro-endocrine disorders in a woman of any age; glandular HPE upon first detection in postmenopausal women.
Endometrial polyps are the most common type of HPE, found in 5.3-25% of gynecological patients of all age groups. Most often, endometrial polyps are detected in pre- and postmenopausal women and malignant in 2-3% of cases.
An endometrial polyp is a benign tumor originating from the basal layer of the endometrium. The glands in the polyp are located unevenly, randomly, have a different size and shape, lined with prismatic epithelium of an indifferent or proliferative type. The vessels have thickened, sclerosed walls; at the base of the polyp they can form tangles. The pathognomonic anatomical sign of the endometrial polyp is its base (“leg”).
Depending on the histological structure, glandular (functional, basal type), glandular-fibrous and fibrous endometrial polyps are distinguished. Adenomatous polyps are referred to as precancerous conditions of the uterine mucosa. Glandular polyps are most characteristic of the reproductive period, glandular-fibrous – for pre- and perimenopause, fibrous-glandular and fibrous – for postmenopause.
In the reproductive and premenopausal periods, endometrial polyps as a histologically independent form can be determined both against the background of HPE and with the normal mucous membrane of various phases of the menstrual cycle.
Postmenopausal endometrial polyps, as a rule, are single, in 20-26% of patients they are multiple. Postmenopausal endometrial polyps are always determined against the background of atrophic mucous membrane, sometimes reaching large sizes. Going beyond the cervix, endometrial polyps mimic polyps of the cervical canal.
Of particular note is the recurrent form of endometrial polyps. The concept of “relapse” is not applicable if hysteroscopic control was not performed before removal of the endometrial polyp.
In recent years, the main role in the occurrence of endometrial polyps has been given to infectious and immune factors. The development of glandular fibrous polyps of the endometrium in 75% of cases occurs with undisturbed hormonal ratios, in 95.3% of patients the endometrium is infected. In addition, in patients with HPE, pronounced immunodeficiency is detected due to the immunosuppressive effect of estrogens, the nature and amount of infectious agents, and the duration of chronic inflammation. This is especially true of the postmenopausal period, when the synthesis of estrogen in the ovaries decreases sharply, metabolic processes in the endometrium and resistance to the action of any damaging factor, including microbial, decrease. As a result of these disorders, a chronic inflammatory process develops in the uterus, which leads to diffuse GGE, and then to the development of focal proliferates.
The role of hormonal disorders as the main factor in the occurrence of endometrial polyps is questioned by many researchers.
The hormonal factor in the pathogenesis of the endometrial polyp is currently being considered in patients receiving tamoxifen. Approximately 8% of breast cancer patients receiving tamoxifen form endometrial polyps.
Symptoms of Endometrial Hyperplastic Processes
Uterine bleeding, often acyclic, contact blood discharge, less often menorrhagia. With large endometrial polyps, there may be cramping pains in the lower abdomen. Sometimes endometrial polyps remain asymptomatic, especially in postmenopausal women.
Since the pathogenetic basis of GGE is anovulation, the leading symptom in patients of reproductive age is infertility, as a rule, primary. The role of endometrial polyps in infertility and miscarriage is debatable.
Diagnosis of Hyperplastic Processes of the Endometrium
The main diagnostic methods for GGE are transvaginal ultrasound, hydrosonography and hysteroscopy. However, the final diagnosis indicating the type of GGE is established after histological examination of endometrial scraping.
A cytological study of aspirate from the uterine cavity is recommended as a screening of the pathology of the endometrium and its condition against the background of hormonal therapy. The method allows you to determine the severity of proliferative changes, but does not give a clear idea of its pathomorphological structure.
Transvaginal ultrasound scanning. Ultrasound with transvaginal scanning is highly informative, non-invasive, harmless to the patient. The information content of the method varies depending on the type of endometrial pathology and the age of the woman. The significance of transvaginal ultrasound increases when combined with hydrosonography.
Diagnostics of GGE with ultrasound is based on the identification of an enlarged anteroposterior midline uterine echo (M-echo) with increased acoustic density. In menstruating women, the thickness of the M-echo should be evaluated in accordance with the phase of the menstrual cycle. It is best to conduct a study immediately after menstruation, when a thin M-echo corresponds to complete rejection of the functional layer of the endometrium, and an increase in the anteroposterior size of the M-echo throughout or locally should be regarded as a pathology. The structure of a hyperplastic endometrium can be either homogeneous or with echo-negative inclusions, which is difficult to differentiate with endometrial polyps. An atypical GGE can be determined, in which, on the echogram, even thickened contours of the endometrium with low sound conductivity limit the homogeneous zone with a lower wave impedance. In most cases, it is not possible to distinguish glandular endometrial hyperplasia from atypical with ultrasound.
In postmenopausal women with a duration of up to 5 years, the thickness of the M-echo up to 5 mm can be considered the norm, with prolonged postmenopausal women, the thickness of the M-echo should not exceed 4 mm (with a uniform structure). In postmenopausal patients receiving HRT, the nature of the M-echo is evaluated depending on the type and regime of HRT and doses of hormonal drugs.
The accuracy of diagnosis of GGE with ultrasound is 60-70%. Hydrosonography does not improve diagnosis.
The ultrasound picture of endometrial polyps shows ovoid, less often rounded inclusions in the structure of the M-echo and uterine cavity with increased echo density. Diagnostic difficulties arise with glandular polyps of the endometrium, which, in accordance with the shape of the uterine cavity, are leaf-shaped, flattened, may not lead to a thickening of the M-echo and are close in sound conductivity to the surrounding endometrium. The registration of color echo signals during a Doppler study allows us to differentiate polyps with intrauterine synechiae, and in menstruating patients with blood clots, but the blood flow with CDC in polyps is not always determined. The information content of transvaginal ultrasound with endometrial polyps is 80-98%. Contrasting the uterine cavity during hydrosonography expands the diagnostic capabilities of ultrasound and allows you to accurately localize the leg of the polyp.
Sonography does not determine the morphological structure of the pathological process in the endometrium. However, high information content along with minimal invasiveness allows I to use transvaginal ultrasound imaging for mass examination, especially postmenopausal women and receiving HRT, as well as for differential diagnosis of various pathological conditions of the uterine mucosa, accompanied by bleeding.
Hysteroscopy. The information content of hysteroscopy in the diagnosis of GGE is 63-97%. Hysteroscopy is necessary both before curettage of the uterine mucosa, to verify the nature and localization of the pathology, and after it to monitor the thoroughness of the operation.
GGE. The hysteroscopic picture depends on the nature of hyperplasia (normal or polypoid), prevalence (focal or diffuse), the presence of bleeding and its duration.
With normal GGE and the absence of blood secretions, the endometrium is usually thickened in the form of folds of various heights, pale pink, swollen, a large number of ducts of the glands are visible (transparent points). When the fluid flow rate changes, a wave-like movement of the endometrium is noted. If hysteroscopy is performed with prolonged blood discharge, fringed scraps of the endometrium of a pale pink color are determined in the bottom of the uterus and in the area of the mouth of the fallopian tubes (the rest of the endometrium is thin, pale). Such a hysteroscopic picture is difficult to differentiate with endometrium in the early proliferation phase. The final diagnosis is made by histological examination of scraping.
In case of polypoid GGE, the uterine cavity was visually performed along the entire length with polypoid growths of the endometrium of a pale pink color, sometimes with vesicles on the surface, multiple endometrial synechiae. The surface of the endometrium with this type of hyperplasia looks uneven, with fossae, cysts, grooves of various sizes. As a rule, changes are more pronounced in the bottom of the uterus and along the back wall. Polypoid GGE, especially when performing hysteroscopy on the eve of menstruation, is difficult to differentiate with endometrium in the phase of late secretion. In such cases, to establish a diagnosis, it is necessary to compare the hysteroscopic picture with the clinical picture of the disease, the day of the menstrual cycle.
Atypical GGE and focal adenomatosis do not have characteristic endoscopic criteria and their hysteroscopic picture resembles ordinary glandular-cystic hyperplasia. In severe atypical HPE, glandular, polypous, dull, overgrowths of a yellowish or grayish color can be determined. More often they are variegated – yellowish-grayish with a whitish bloom. As a rule, the final diagnosis is established after histological examination.
Hysteroscopy fibrous polyps of the endometrium are determined in the form of pale single formations, round or oval in shape, a bowl of small size (from 0.5×1 to 0.5×1.5 cm), usually on a leg, of dense structure, with a smooth surface, malovascularized. Sometimes fibrous polyps of the endometrium reach large sizes, and with hysteroscopy, the surface of the polyp, which is tightly attached to the uterine wall, is mistakenly considered an atrophic mucous membrane and the polyp is not diagnosed. If a polyp is found, it is necessary to examine it from all sides, to evaluate the size, localization, place of attachment, the size of the legs. Fibrous polyps resemble submucosal myomatous nodes.
Glandular cystic polyps of the endometrium, unlike fibrous ones, are more often large (from 0.5×1 to 5×6 cm), single, although there may be several polyps. The shape of the polyps is oblong, conical, irregular (with jumpers), the surface is smooth, even, in some cases cystic formations with a thin wall and transparent contents protrude above it. The color of the polyps is pale pink, pale yellow, grayish pink. Often the tip of the polyp is dark crimson or cyanotic-crimson. On the surface of the polyp are visible vessels in the form of a capillary network.
Adenomatous polyps of the endometrium are often localized closer to the mouths of the fallopian tubes and, as a rule, are small (from 0.5×1 to 0.5×1.5 cm), they look dull, gray, loose. Adenomatous changes can also be determined in the tissue of glandular cystic polyps, in this case, the nature of the polyp during endoscopic examination cannot be determined.
Endometrial polyps change shape when the flow rate of fluid or gas into the uterine cavity changes. In this case, the polyps are flattened, increase in diameter, and when the pressure decreases, they are extended in length and make oscillatory movements.
Histological examination of scrapings of the uterine mucosa is the method of final diagnosis of GGE.
Treatment of Endometrial Hyperplastic Processes
The treatment of HPE depends on the pathomorphological characteristics of the endometrium, the patient’s age, etiology and pathogenesis of the disease, concomitant gynecological and extragenital pathology.
Therapy at various age periods consists of stopping bleeding, restoring menstrual function in the reproductive period or persistent menopause at an older age and preventing the relapse of the hyperplastic process.
Management of patients of reproductive age with GGE. The traditional method of treating HPE is hormone therapy. In her reproductive age, she pursues the goal of both preventing the recurrence of HPE and restoring the ovulatory menstrual cycle.
With GPE without atypia and glandular polyps of the endometrium, combined estrogen-progestogen drugs or the so-called combined oral contraceptives (COCs) are used according to the contraceptive scheme. Preference is given to preparations containing 3rd generation progestogens with a lower frequency of androgenic type adverse reactions and not giving metabolic effects (Silest, Regulon, Novinet, Rigevidon).
In late reproductive age, it is preferable to use progestogen drugs Primolut-nor, Norcolut, Duphaston, 5-10 mg test from the 16th to the 25th day of the menstrual cycle for 6 months; 17-OPK 250 mg IM on the 14th and 21st day of the cycle for 3-6 months.
Monitoring the effectiveness of treatment is carried out by aspiration endometrial biopsy and ultrasound after 3, 6 and 12 months. In doubtful cases or if a pathological process of the endometrium is suspected, it is advisable to conduct hysteroscopy with separate diagnostic curettage of the uterine mucosa.
Clinical observation is carried out for at least 1 year with persistent normalization of the menstrual cycle.
With GGE with atypia and adenomatous polyps of the endometrium in patients of reproductive age, hormonal treatment is carried out continuously with progestogens or antigonadotropic drugs:
- zoladex, diferelin 3.6 mg s / c once every 28 days, 3 injections;
- buserelin (endonasal spray), 3 times a day (0.9 mg / day), for 6-9 months;
- gestrinone, non-country 2.5 mg 2-3 times a week, for 6-9 months;
- 17-OPK 500 mg IM twice a week for 6–9 months;
- danazol, gave 400-600 mg daily for 6-9 months.
To assess the effectiveness of hormonal therapy of HPE after 3, 6 and 12 months from the start of treatment, a follow-up examination is shown, including ultrasound and cytological examination of the uterine aspirate. It is advisable to carry out a diagnostic diagnostic curettage of the uterine mucosa with hysteroscopy 3 months after the start of treatment.
In order to form an ovulatory menstrual cycle in young women, ovulation stimulants are further shown (clomiphene from 50 to 150 mg per day from the 5th to the 9th day of the cycle for 3-6 months). After normalizing the menstrual cycle, treatment is stopped.
Clinical observation should be carried out within 12-24 months after discontinuation of treatment in the absence of data indicating endometrial pathology.
The recurrence of GGE indicates inadequate therapy or hormone-active structures in the ovaries, requires clarification of their condition, including visual diagnostic methods (ultrasound, laparoscopy, biopsy or resection of the ovaries). The absence of morphological changes in the ovaries allows continuing hormone therapy with higher doses of drugs. Infectious factor should also be excluded as a possible cause of GGE and hormone therapy inefficiency.
With the ineffectiveness of hormone therapy, the recurrence of GGE without atypia, ablation (resection) of the endometrium is indicated. Necessary conditions for performing a hysteroscopic operation: unwillingness of a woman to become pregnant in the future, age over 35 years old, desire to save the uterus, uterus size not exceeding (according to different authors) 10 weeks of pregnancy. Myoma is not a contraindication to ablation (resection) of the endometrium, but none of the nodes should be more than 4-5 cm. Adenomyosis worsens the results of the operation.
Re-occurrence of atypical GGE or adenomatous polyps of the endometrium requires surgical treatment (the volume of surgery is decided individually).
Treatment of GGE in patients in pre- and perimenopause. The first stage of treatment also includes hysteroscopy with separate diagnostic curettage of the uterine mucosa. Further therapy depends on the morphological structure of the endometrium, concomitant gynecological and extragenital pathology. The choice of a hormonal drug, the scheme and duration of treatment are also determined by the need to maintain a rhythmic menstrual-like reaction (up to 50 years) or a persistent cessation of menstruation.
Hormone therapy of GGE without atypia and glandular polyps of the endometrium involves the use of progestogens in a cyclic or continuous mode (to stop menstruation):
- primolut-nor, norcolut, 10 mg test from the 5th to the 25th day of the menstrual cycle (with the menstrual cycle preserved) or daily for 6 months;
- depot check at 150 mg IM once a week for 6 months;
- 17-OPK 250 mg IM on the 14th and 21st day of the menstrual cycle or 2 times a week for 6 months;
- buserelin (endonasal spray) 3 times a day (0.9 mg / day) for 6 months;
- zoladex, diferelin 3.6 mg sc 1 time in 28 days, 3-4 injections.
Monitoring of the effectiveness of treatment is carried out by ultrasound after 3, 6 and 12 months, by aspiration endometrial biopsy after 3 months and hysteroscopy with separate diagnostic curettage of the uterine mucosa with indications of endometrial pathology (after the initial examination by screening methods).
Clinical observation is carried out for at least 1 year with persistent normalization of the menstrual cycle or persistent postmenopause.
HPE with atypia in perimenopause requires the appointment of higher doses of parenteral progestogens or GnRH analogues:
- zoladex, diferelin at 3.6 mg sc 1 time in 28 days, 4-6 injections;
- buserelin (endonasal spray) 3 times a day (0.9 mg / day) for 6-9 months;
- gestrinone, non-mestran 2.5 mg 3 times a week for 6-9 months;
- Depot-test 300-600 mg / m once a week for 6-9 months;
- 17-OPK 500 mg IM twice a week for 6–9 months;
- danazol, gave 600 mg orally daily for 6–9 months.
Monitoring the effectiveness of treatment is carried out 3 months after the start of treatment by aspiration endometrial biopsy, ultrasound. After 6 months, a separate diagnostic curettage of the uterine mucosa under the control of hysteroscopy is indicated. Clinical observation is carried out for 12-24 months with dynamic ultrasound screening control.
With recurrent GGE without atypia, the inability to conduct hormone therapy due to concomitant extragenital pathology, a hysteroscopic operation is shown – ablation (endometrial resection). Relapse of HPE, as well as a combination of this pathology with uterine myoma and / or internal endometriosis in patients with pre- and perimenopause, require an expansion of indications for radical surgery (hysterectomy).
Management of postmenopausal patients with GGE. With the first detected GGE in postmenopausal women, it is advisable to administer hormone therapy with prolonged gestagens (17-OPK, depot-test) in continuous mode for 8-12 months or GnRH analogues (buserelin, zoladex, diferelin) for 6-8 months in parallel with hepatoprotectors , anticoagulantamia, antiplatelet agents. Treatment is carried out under ultrasound monitoring of the condition of the pelvic organs and cytological control after 3 and 6 months. Separate diagnostic curettage with hysteroscopy is indicated for those patients who are suspected to have endometrial pathology during a screening examination.
Relapse of GGE in postmenopausal women is an indication for surgical intervention – hysteroscopic ablation of the endometrium or hysterectomy with appendages. Acceptable supravaginal amputation of the cervix with appendages (in the absence of cervical pathology) or bilateral adnexectomy.
With atypical GGE in postmenopause, it is immediately necessary to solve the question of radical surgery – panhisterectomy. With severe extragenital pathology and a significant risk of surgical treatment (panhisterectomy), prolonged treatment with the above hormonal drugs is permissible.
Treatment of patients with endometrial polyps. The main treatment is targeted polypectomy. Complete removal of the endometrial polyp (with the basal layer of the endometrium in place of the polyp) is possible only with the help of hysteroscopic equipment. For polypectomy, you can use both mechanical endoscopic instruments, and electrosurgical technology, a laser conductor. Electrosurgical excision with hysteroscopy is recommended for fibrotic and parietal, as well as for recurrent polyps of the endometrium. In perimenopausal patients, it is advisable to combine a hysteroscopic polypectomy with endometrial ablation (resection).
There is no consensus on the tactics of further conduct. Many authors believe that removing the polyp is enough. Others after removal of glandular and glandular-fibrous polyps of the endometrium prescribe hormone therapy. The type and duration of hormone therapy depends on the age of the patient, the morphological structure of the polyp, and concomitant pathology. In the treatment of patients with endometrial polyps in postmenopausal women, the same hormonal drugs are used as in other types of HPE in this age group.